| | | | | | | | S | M | T | W | T | F | S |
|
1 |
2 |
3 |
4 |
5 |
6 |
| 7 |
8 |
9 |
10 |
11 |
12 |
13 |
| 14 |
15 |
16 |
17 |
18 |
19 |
20 |
| 21 |
22 |
23 |
24 |
25 |
26 |
27 |
| 28 |
29 |
30 |
31 |
|
| | | | | | Basic Instructions Before Leaving Earth |  | But the fruit of the Spirit is love, joy, peace, forbearance, kindness, goodness, faithfulness. |
|
|
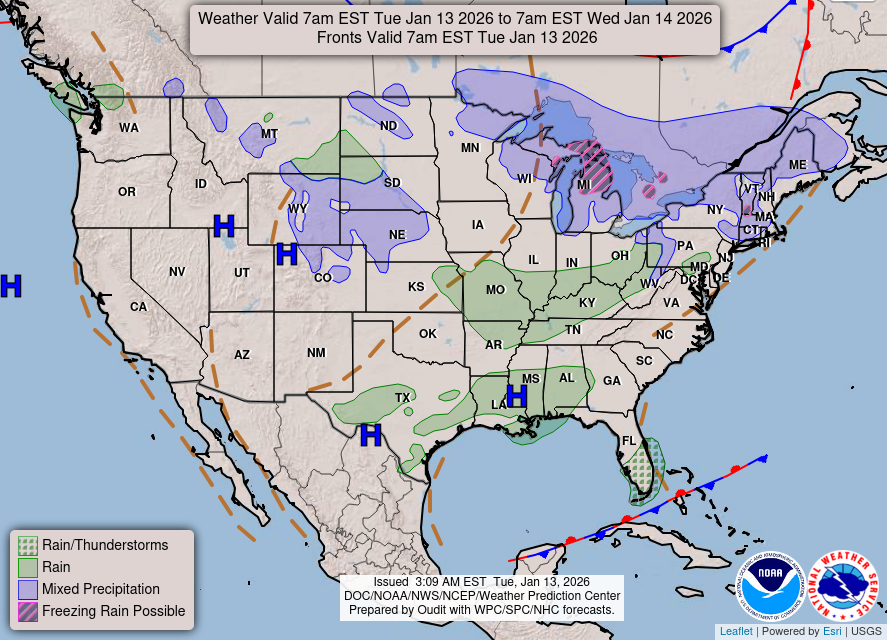
| | | | | | | |  | World Tide |
| Overnight

Low: 57 °F | Clear
|
| Saturday

High: 85 °F | Sunny
|
|  | 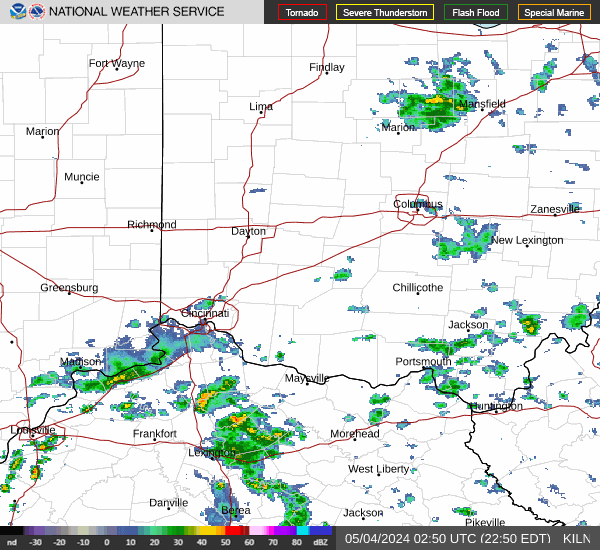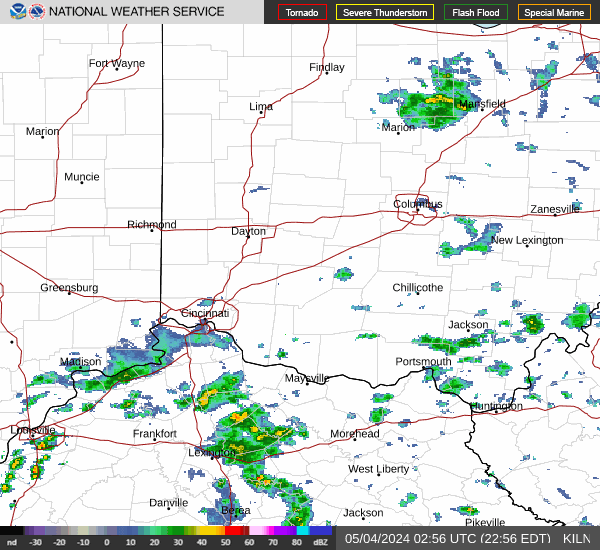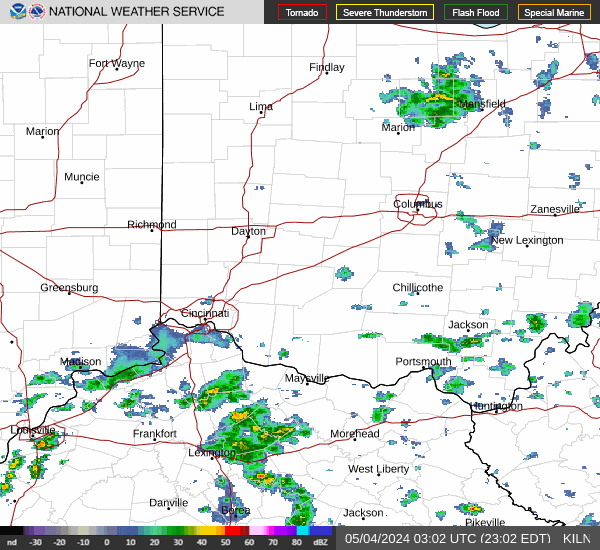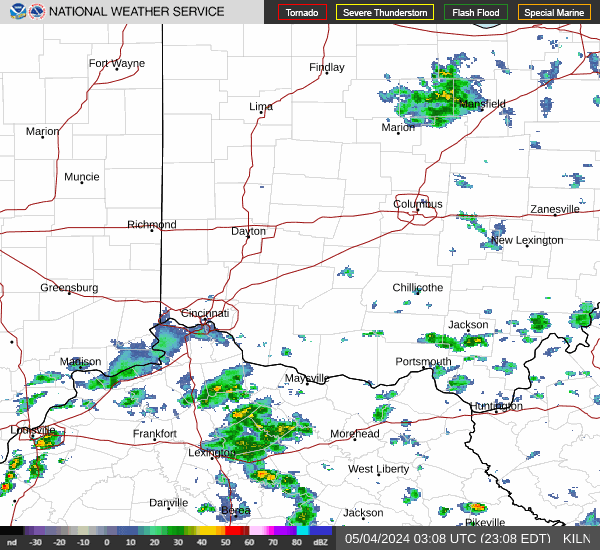 |
| |  | Bitcoin Hopper | | | Market Cap: $2,413,564,080,921 | 24H Volume: $61,723,836,419 |
|
| | Status | Price | Height | Block
Reward | Workers
Shares | Last Block
Time Since Last | Pool |  | | $67,831.89 | 850000 | 3.125 BTC | | | |  | | $380.91138791 | 856272 | 3.125 BCH | 0
0% |
0:0 | |  | | $0.00756200 | 19699019 | 339 DGB | 0
14.77% | 19696491
10:29 | |  | | $0.13442421 | 5309448 | 10000 DOGE | 0
0% |
0:0 | |
| |  Create Thread Create Thread |
| Subject / Message | Started by | View | Last Post | Option |
|
|

 Register Now! - [ Click Here ]
Register Now! - [ Click Here ]